Abstract
Introduction Over the last decade, recurrent oncogenic alterations in the MAPK/ERK pathway have been discovered in various histiocytic and dendritic cell disorders, especially Erdheim-Chester disease (ECD), Langerhans cell histiocytosis (LCH), and Rosai-Dorfman disease (RDD). These discoveries have led to the successful use of targeted BRAF- and MEK-inhibitors. However, most data are derived from small institutional studies mostly focusing on ECD and LCH, which leaves room for a comprehensive evaluation of the molecular underpinnings in a large cohort.
Methods Formalin-fixed paraffin embedded tumor biopsies from various histiocytic/dendritic cell disorders seen at a tertiary institution from 2001-2021 were subjected to whole exome sequencing (WES) using one of two assays: in-house SureSelectXT AllExon V5+UTR or commercially available Tempus xT assay. Identified variants were filtered to remove polymorphic, low coding impact and low read coverage variants. A variant was then selected for further analysis if it met one of the following criteria: 1) observed as somatic in ≥1 tumor-germline pairs or COSMIC database or 2) observed in at least three samples with an allele frequency of ≥10% in ≥1 sample. Cases were clustered into mutually exclusive groups based on their primary driver mutational profile using the following funneling order: BRAF-V600E (BRAF-V600E mutations), BRAF*/NRAS/KRAS (BRAF non-V600E, KRAS, or NRAS mutations), CSF1R (CSF1R mutations), MAPK (MAPK/MEK mutations), and PI3K (AKT3, INPP4B, MTOR, PIK3CA, PIK3R1, PIK3R2, PIK3R3, PPP2R1A, MTR mutations). A cancer specific pathway enrichment was performed on the unknown cases, which identified epigenetic mutations as major pathway alterations observed in the cohort. We also correlated the presence of epigenetic mutations with clinical features and outcomes.
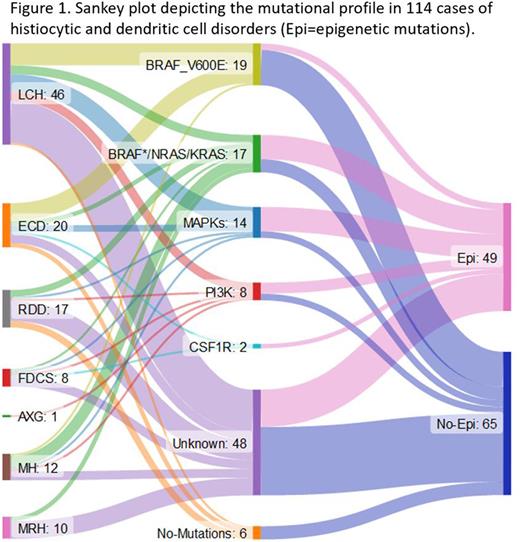
Results We included 114 cases with histiocytic and dendritic cell disorders in the study. Median age at diagnosis was 51y (IQR 37-64). The disease distribution was as follows: 46 LCH, 20 ECD, 17 RDD, 8 follicular dendritic cell sarcoma (FDCS), 1 adult xanthogranuloma, 12 malignant histiocytosis (MH; including histiocytic and Langerhans cell sarcoma), and 10 multicentric reticulohistiocytosis (MRH). Based on the known driver mutations in histiocytoses, the following 5 molecular groups were identified: BRAF-V600E (n= 19, 17%), BRAF*/KRAS/NRAS (n=17, 15%), MAPK (n=14, 12%), PI3K (n=8, 7%), CSF1R (n=2, 2%) (Figure 1). A large proportion (n=48, 42%) did not have any of the known driver mutations, while 6 cases (5%) had no alterations identified. Cancer-specific enrichment analysis in the entire cohort revealed mutations in epigenetic genes in 47 (41%) cases, including 30 (26%) cases with identifiable driver mutations and 17 (15%) cases without known driver mutations. Median number of epigenetic mutations was 1 (range 1-11); 21% cases had 2 and 10% cases had 3 epigenetic mutations, respectively. The most common epigenetic mutations included those in KMT2C (34%), KMT2D (23%), and ARID1A (19%).
The mutational groups with highest prevalence of epigenetic mutations were CSF1R (100%), MAPK (71%), and PI3K (63%), while lowest prevalence of these alterations was found in BRAF-V600E group (16%; p=0.0003). The epigenetic gene mutations were seen more commonly in MRH (90%) and MH (50%) compared with ECD (45%), RDD (41%), FDCS (38%), and LCH (33%; p=0.037). There was no association between epigenetic gene mutations and age at diagnosis or organ involvement. The median overall survival (OS) was not significantly different between those with epigenetic mutations and those without such alterations (22y vs. 17y, p=0.89).
Conclusions In this large study of patients with diverse histiocytic and dendritic cell disorders, we found a high prevalence of mutations in epigenetic genes (41%). These mutations were specifically enriched in tumors with non-BRAF-V600E mutations, and in MRH/MH. These data shed light on additional mechanisms of disease pathogenesis, and with available epigenetic therapies, warrant further investigation.
Disclosures
Goyal:UpToDate: Patents & Royalties; SeaGen: Research Funding; Viracta Therapeutics: Research Funding; Sutro Biopharma: Research Funding; 2nd.MD: Consultancy. Vassallo:Bristol Myers Squibb: Research Funding; Sun Pharma: Research Funding; Pfizer: Research Funding. Shah:Astellas: Research Funding; Celgene: Research Funding; Marker Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal